Electronic spectroscopy is a powerful method for investigating transition metal complexes. Additional and complementary information can be provided by magnetic measurement because complexes generally have partially filled metal d or f orbitals so a large range of magnetic properties can be expected depending on oxidation state, e– configuration & co-ordination number of central atom.
DIAMAGNETISM
- When a substance is placed in an external or applied magnetic field, there is an induced circulation of e– in substance, this induced circulation of e– give rise to a magnetic moment or magnetic field and if this opposes the applied magnetic field then the substance is repelled by the magnetic field.
- This effect is called Diamagnetic effect.
- This effect is caused by the presence of paired electron.
- The diamagnetic effect exists only when a substance is placed in the magnetic field.
- The substances that have only paired electron exhibits a weak magnetic moment called Diamagnetism and such substances are called Diamagnetic Substance.
“If a substance has only paired e–, the diamagnetic effect is dominated while if a substance has paired as well as unpaired electron, the paramagnetic effect is dominated”.
“Diamagnetism is a universal property of matter since all compound contains some paired electron”

PARAMAGNETISM:-
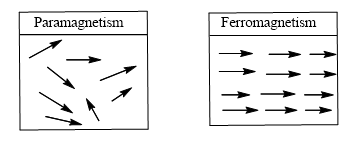
- Any substance that has one or more unpaired e¬– exhibits stronger & permanent magnetic property known as paramagnetism
- Paramagnetism arises from spin and orbital motion of unpaired e– in the absence of external magnetic feild
When a paramagnetic substance is placed in an external magnetic field, the permanent magnetic moments tends to align themselves in the direction of external magnetic field and as a result they are attracted into the magnetic fields.
Since, paramagnetic effect in much large than diamagnetic effect & opposite to diamagnetic effect, it cancels the diamagnetic effect.
- Thus even substances having only one unpaired electron per molecule will show a net attraction into a magnetic feild.
The paramagentic effect is observed only in the persence of an external field. When field is removed, individual molecular moments are randomised by thermal motion and bulk sample has no overall moment. When a field is present there is a competition between the thermal tendency towards randomness & field capacity to force alignment. As a result, paramagnetic effects decrease in magnitude as temperature is increased. It is due to the fact that increase in temperature, increase randamization and decreases paramagnetic effect
Now, When any substance is placed in a magnetic field, the field produced within the sample will either be greater than or less than applied field, depending on whether the material is paramagentic or diamagnetic.
The difference between the two is expressed as:-
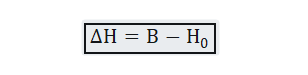
Where B is induced field inside the sample, is external field or free field value

More commonly, the difference between the applied and induced field in sample is expressed in terms if I (ie. intensity of magnetisation), which is magnetic moment per volume
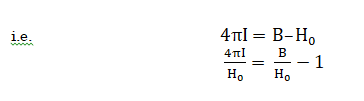
![]()
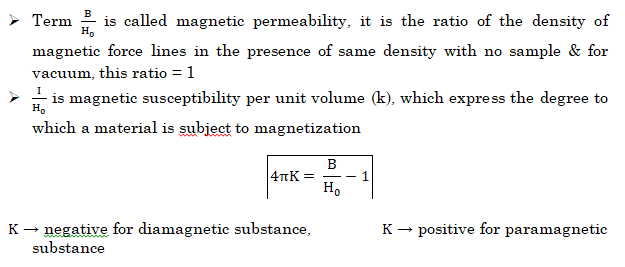
The quantity that is most frequently obtained from experimental measurement for magnetism is specific or mass susceptibility.
It is related to volume susceptibility through density (d)
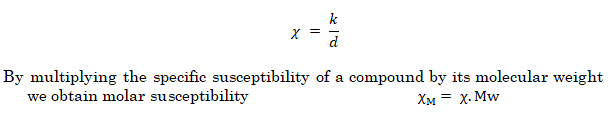
- Methods in laboratory for the measurement of magnetic susceptibility
(a) Gouy Method
(b) Faraday Method
MORDERN TECHNIQUE:- SQUID (Super conductor quantum interference device)
- Once an experimental value of has been obtained for a paramagnetic substance it can be used to determine how many unpaired e– are there per-molecule or ion.
- Next step is to connect the macroscopic susceptibility to individual molecular moment & finally to unpaired electron.
- From classical theory, the corrected or paramagnetic molar susceptibility is related to permanent magnetic moment of molecule (μ).
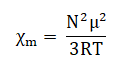
Where, N is Avagadro number
R is ideal gas constant
T is absolute temperature
μ is expressed in Bohr Magneton (1BM= eh/4πm)
Now, solving this expression for magnetic moment we get:-
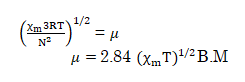
- Pierre curie has shown that the paramagnetic susceptibility is inversely proportional to absolute temperature.
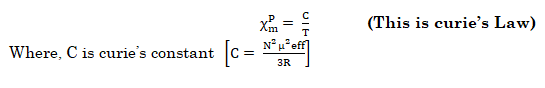
This law is valid only for paramagnetic substances that are magnetically dilute (those in which paramagnetic centers are well separated from each other) by diamagnetic atoms.
- NOTE:- Magnetic susceptibility χ of a material is a measure of how easy it is to align electron spins with the applied field.
- A paramagnetic material has positive susceptibility.
- A Diamagnetic material has negative susceptibility.
Cooperative Mechanism
- The materials that are not magnetically dilute, unpaired spins on neighboring atoms may couple with each other, this phenomenon is called Magnetic Exchange.
- These substance may behave as either ferromagnetic or ferromagnetic or antiferromagnetic and they follow Currie-Weirs Law (modification of curie’s Law) (This is called cooperative magnetism).
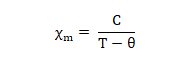
Where, Θ is a constant with units of temperature.
- NOTE– When the paramagnetic species are very close together (as in bulk metal or are separated by species that can transmit interactions (as in many d-block metal oxides flourides & chlorides), the metal centers may interacts (couple) with one another, this give rise to ferro or anti-ferro.
- Note: The Paramagnetism is hot topic for “CSIR NET Coaching in Chemistry”.
Ferromagnetism:-
- If the interacting magnetic dipoles on neighboring atom tends to assume a parallel alignment, substance is said to be ferromagnetic.
- Ferromagnetism leads to greatly enhanced para magnetism as in iron metal at temperature of up to 1043 K above which thermal energy is sufficient to overcome the alignment and normal paramagnetic behavior prevails
- On cooling a sample, ferromagnetic ordering (change from paramagnetic to ferromagnetic occurs at curie temperature and curie’s weirs law is written as:-
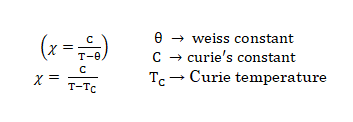
- If there is interaction between the magnetic moment on neighboring atoms of a paramagnetic substance, spontaneous ordering of magnetic moment occur below a particular temperature, called as critical temperature.
- If alignment of all the magnetic moment of neighboring atoms is in same direction & produce a permanent magnetic moment substance is said to be ferromagnetic and critical temperature is called curie temperature ( Tc) .
Above this temperature, the substance behaves as a normal paramagnetic substance below Tc, the ferromagnetic substance obeys Curie Weiss law,
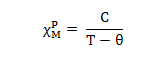
For example Fe, Co, Ni & CrO2 exhibit ferromagnetism below curies temperature (1043K, 1404K, 631K & 386K)
Note: Paramagnetism and Ferromagnetism are very useful for “GATE COACHING in Chemistry”.
Antiferromagnetism
If there is a tendency for anti–parallel arrangement of coupled spins, then the substance is said to be Antiferromagnetic.
If half of the magnetic moments are alighned in opposite direction to the other half resulting in a net zero magnetic moment, the substance is said to be anti–ferromagnetic and the critical temperature is called Neel Temperature.
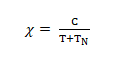
Where, TN is Neel temperature
An example of Antiferromagnetism is MnO which has NaCl type structure & has Neel temperature of 118K

Ferrimagnetism
If some of the magnetic moments are systematically aligned opposite to the others to give resultant magnetic moment and the critical temperature is called Curie temperature T_C.
Ex. Magnetite (Fe3 O4), In this substance, the magnetic moments of Fe(II) & Fe(III) are aligned in opposite directions and resultant magnetic moment is only from Fe(II) moment.

Plot of reciprocal of paramagnetic susceptibility v/s absolute temperature

Variation of magnetic susceptibility with temperature for diamagnetic, paramagnetic, ferromagnetic, and antiferromagnetic substances. Transition to paramagnetic behavior for ferromagnetic and antiferromagentic substances occur at the Curie (Tc) and Neel (TN) temperatures, respectively.
Note: Paramagnetism, Ferromagnetism, Antiferromagnetism and Ferrimagnetism are essential topics for “JAM COACHING in Chemistry”.
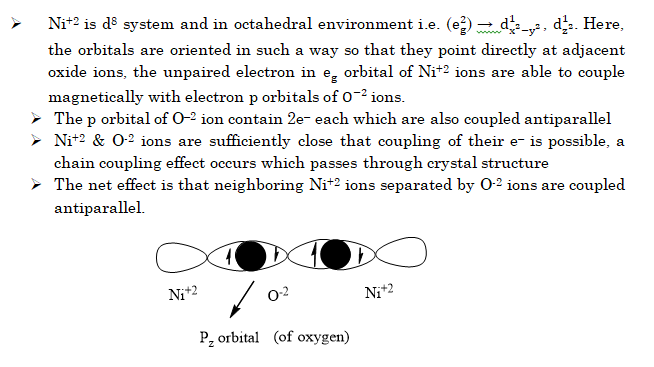
Super exchange:- When a bridging ligand facilitates the coupling of electron spins on adjacent metal centers, the mechanism is called super exchange.
In super exchange pathway, the unpaired e– on the first metal centre M1 interacts with a spin–paired pair of e– on bridging ligand with the result that unpaired e– on M2 is aligned in an antiparallel manner with respect to M1.

Where, M is magnetization produced, H is Field applied
Ex. MnO, NiO occurs to give rise to Anti ferromagnetism)
The above mentioned topic is essential for CSIR-NET Coaching in Chemistry. It is a hot topic for “GATE Coaching in Chemistry” and “JAM COACHING in Chemistry”