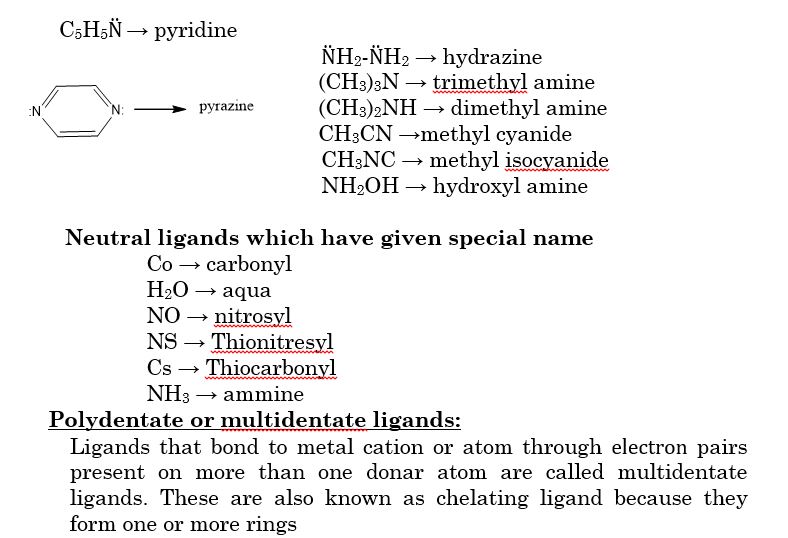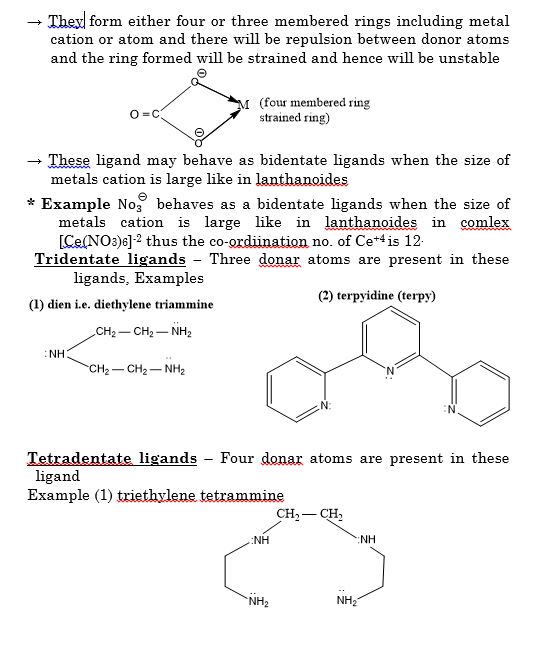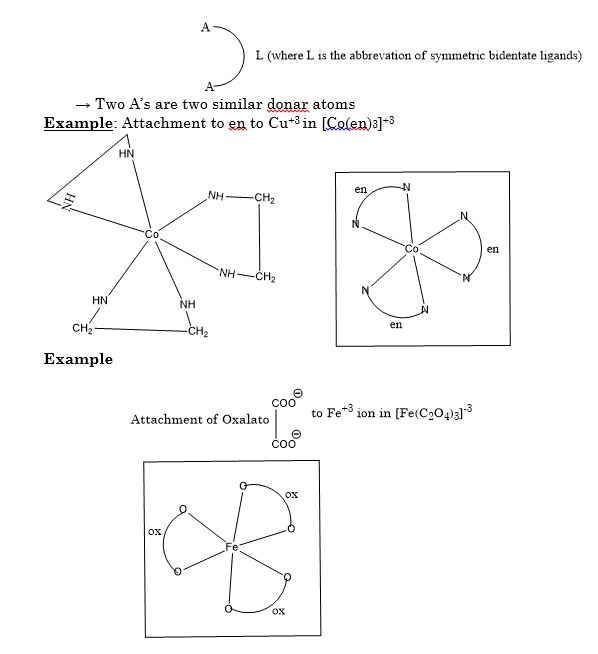
The branch of Inorganic Chemistry that deals with the study of coordination compounds is called coordination chemistry.
A compound in which a metal cation or atom is attached to a group of ligands by coordinate bond is called co-ordination complex.
Ligand: Any species (ion or molecule) that have atleast one lone pair of electrons and have tendency to donate it to metal cation or atom. It is electron rich species that is why it is called as Lewis base (or nucleophile).
Metal: It is an electron deficient species which can accept a pair of electrons. Therefore, it behaves as lewis acid or an electrophile. A group of ligands donate its pairs of electrons. One donor atom of a ligand donates to metal cation to form coordinate bond. The product so formed is known as coordination compounds. These compounds may be neutral or ionic compounds as: